Check out these amazing flyers about Covid-19 Vaccine development and potential side effects from Alexandra Wickson.

Vaccine Development (/wp-content/uploads/2021/02/Covid-VaccineDevelopment.pdf) 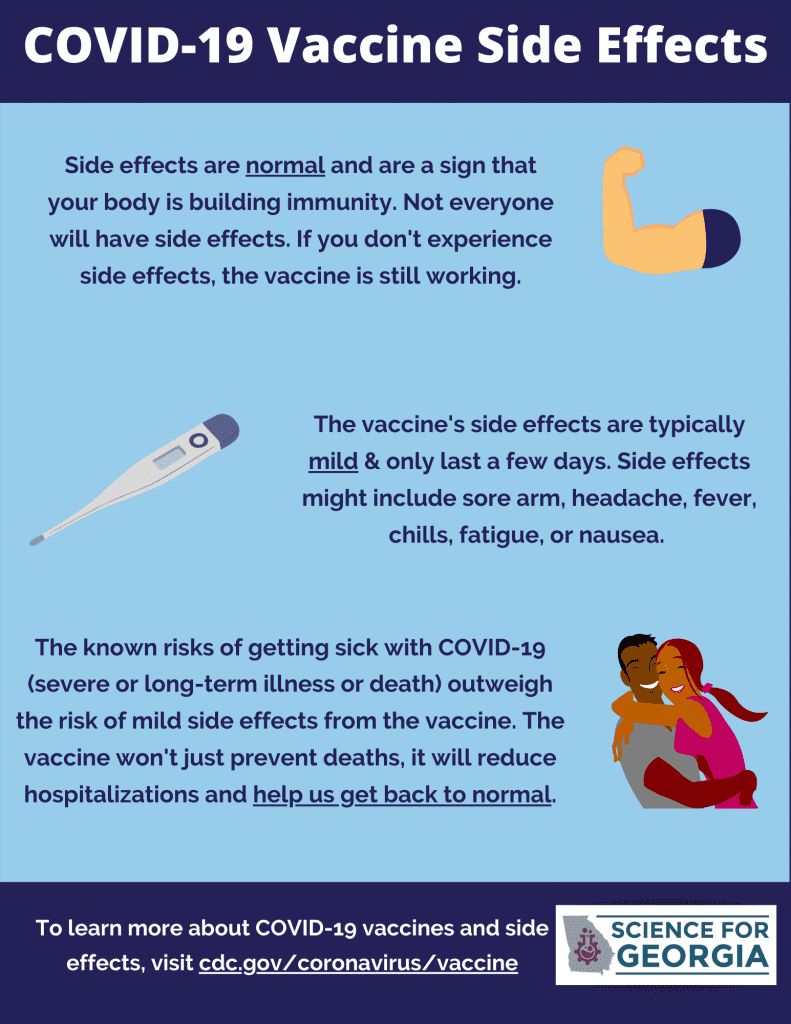
Potential Side Effects (/wp-content/uploads/2021/02/Covid-SideEffects.pdf)
What is an mMRA vaccine?
by Sarah Strassler
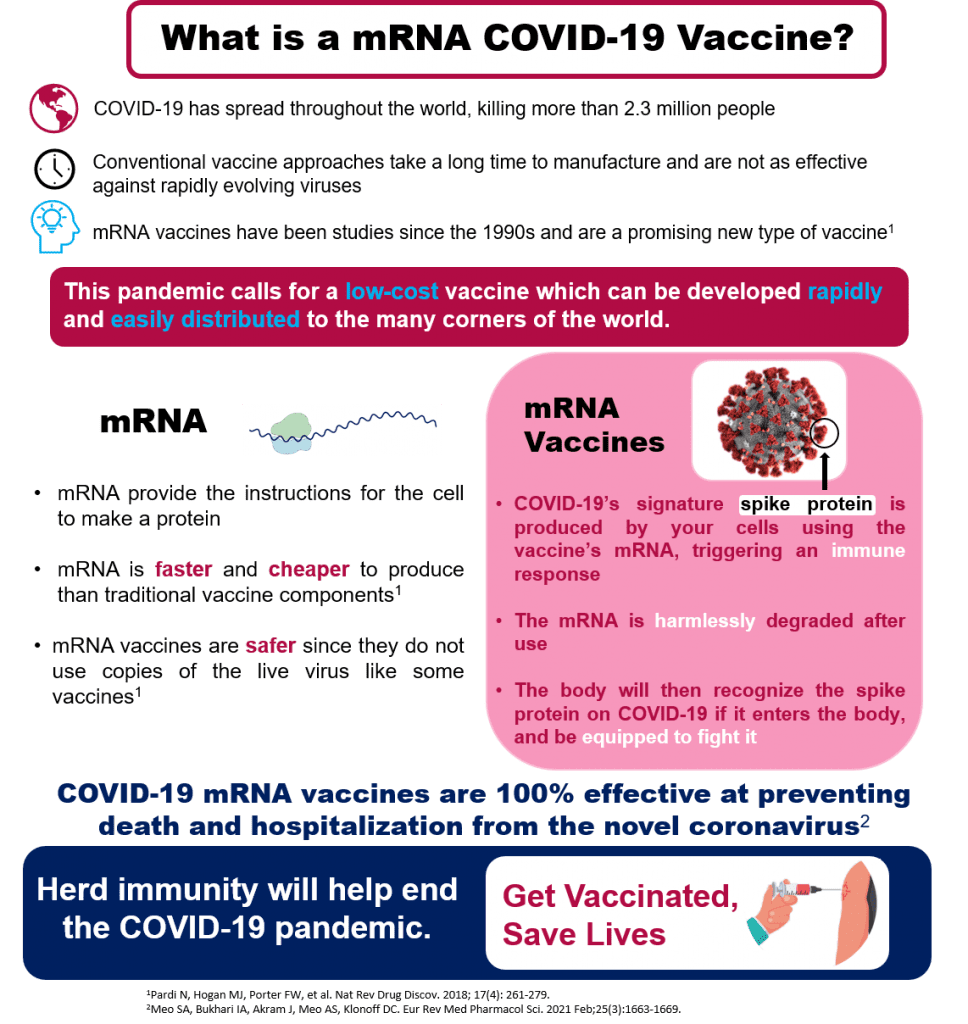
COVID-19 has spread throughout the world, killing more than 2 million people. The most promising solution for an end to this global pandemic is the creation of a highly potent, low-cost vaccine which can be developed rapidly and easily distributed to the many corners of the world. Typical vaccines are developed from preexisting forms of the virus, but these types of vaccines take a long time to manufacture and are not as effective against rapidly evolving viruses. An end to this global pandemic requires an innovative, new approach to vaccine development.
When something enters our body (food, dust, viruses) it is digested, used, or cleaned out by proteins. All living cells produce proteins by using the recipes for them contained in mRNA. Since the 1990s, scientists have been studying ways to use mRNA to train cells to produce a desired protein. The COVID-19 vaccine uses mRNA by causing our own cells to produce the signature spike protein from the COVID-19 virus (that ball with spikes we’ve all seen). The spike protein is “foreign” to the human body and triggers an immune response training our body’s cells to recognize and fight the coronavirus. The mRNA has no long-term effects and is harmlessly degraded after use, leaving only a trained immune response. mRNA vaccines are faster and cheaper to produce than traditional vaccines, and safer for patients since they are not produced using infectious elements.
COVID-19 vaccines developed using mRNA from both Pfizer and Moderna have proven to be 100% effective at preventing death and hospitalization from the novel coronavirus. In order to reach herd immunity and reach an end to the global pandemic, it is important to get vaccinated against the COVID-19 virus when the vaccine becomes available to you.
What are effective ways to talk to someone who is vaccine hesitant?
“Just do it” may be effective for buying some sneakers, but for someone who is generally concerned about vaccines, that isn’t going to work, and it’s also not nice.
Here are some ways to be polite and respectful of their concerns:
- Ask the person if they are willing to talk to you about their concerns.
- Ask them to share what concerns them. Take the time to listen.
- Acknowledge anything they are already doing to stay safe (mask wearing, social distance, etc.).
- Acknowledge their concerns with empathy and understanding. A great way to do that is repeat back what you heard. (i.e. “So if I hear you correctly, you are concerned because you have heard about all the bad side effects…”)
- Ask them if they are open to hear your responses or information to their stated concerns. Don’t just launch into a long-winded response. Frame it around what they told you.
- Always leave the door open for further conversation.
So Would You Personally Get This Science for Georgia?
Here is a personal story from Brandy Wade, Secretary of Science for Georgia, about her personal vaccine decision journey.
As a scientist I approach the decision to receive a novel treatment by looking at the science. As a scientist, I am also frequently asked questions about the new Pfizer and Moderna vaccines. It seems like every other day I field questions like, “is it safe? It seems like the development was rushed”, and “why should I take it if it doesn’t prevent you from getting the virus?”, and “what’s an mRNA?”. So, let me walk you through my decision to get vaccinated and answer some of your burning questions.
What even in mRNA and what’s it doing in these vaccines? An mRNA or messenger RNA is a single strand of genetic material that acts like an instruction manual or blueprint to build a protein that serves some function in a cell or organism. In the Pfizer and Moderna vaccines, the mRNA instructions allow your body to make a small section of the Covid-19 spike protein. The spike protein is essential for the Covid-19 virus to enter a cell and cause infection but won’t cause a real covid infection. When you receive these vaccines you receive this mRNA blueprint that instructs your body to make this very small part of the virus and this protein which, once made, triggers your immune system leading to a cascade that teaches your body to spot and attack this protein. Then, because mRNA’s don’t last very long, the mRNA degrades, having taught your immune system a new trick in no time at all.
But mRNA vaccines are new, right? The Pfizer and Moderna vaccines are the first mRNA vaccines to come to market, but this technology has been in development for over a decade and has undergone extensive testing to date. So even though an mRNA vaccine is new to the market, it’s not new to scientists. The investigators who created the Pfizer and Moderna vaccines built on years of research to create these vaccines in record time.
How did they make these vaccine doses so fast? Doesn’t it take months to make vaccine doses? The nature of mRNA also allows faster synthesis of vaccines and doesn’t require months longs growth of vaccine components in eggs.
Are the vaccines safe? Clinical trials are designed to test the safety and effectiveness of new treatments in a stepwise fashion. Phase 1 clinical trials test the safety in a small number of people. Phase 2 clinical trials tests is the therapy actually works and Phase 3 clinical trials test how well a therapy works as well as ensuring safety in a larger number of people. A good example of this stepwise system at work is the Merk vaccine. Merk recently ended tests on their covid-19 vaccines because ongoing clinical trials demonstrated it didn’t actually provide immunity that well.
Though the timeline for testing of the Moderna and Pfizer vocid-19 vaccines has been compressed, safety wasn’t sacrificed to get these vaccines to market. Traditionally, phase 3 vaccine clinical trials will enroll 10,000 to 15,000 patients and follow up with patients anywhere between 7 days to 2 months after receiving a vaccine dose. By the time Pfizer sought Emergency Use Authorization by the FDA, over 18,000 patients had received both doses of the vaccine and had follow ups 7 days after each vaccine dose. The Moderna vaccine had already been administered to over 14,000 patients and followed up with them for at least 28 days after their final dose. Even though these vaccines have received emergency use authorization from the FDA both trials will continue to enroll new patients and monitor patients for the next two years. Enrolling this many people in a clinical trial usually takes several years, but these trials managed to meet huge enrollment goals in a short amount of time because of the nature of the ongoing pandemic. So, while they did these trials fast, they still prioritized safety. A good example of this system at work is the Merk
Why should I take it if it doesn’t prevent you from getting the virus? Many people quickly noted that the Pfizer and Moderna press releases say the vaccines are effective at preventing covid disease, but they don’t say they are preventing you from getting infected with the virus at all. This is largely based in the way the clinical trials were designed. In order to definitively state that a vaccine prevented you from getting the virus, the clinical trial would require regular testing to see if the enrolled patients had contracted the virus. With limited availability of covid-19 tests would make it difficult to do this so the trials were designed to only test enrolled patients if they had any symptoms of covid. The data they have doesn’t allow them to confidently say you can’t get the virus, but the data does show that the vaccines reduced hospitalizations, prevented deaths caused by Covid-19, and reduced the number of symptomatic cases. The vaccines may actually reduce your chance of getting Covid-19, but since they didn’t directly test for it, they can’t say that is does. But reducing the number of hospitalizations is an important step needed right now with a hospital system that is groaning under the pressure of the ongoing pandemic.
That’s all well and good, but would you get the vaccine? All of the above isn’t just talk. When I got the chance, I did get the vaccine. I received my first dose of the Moderna vaccine on December 30th. For a few days after that first dose, I had a bit of soreness, swelling and redness at the sight of injection. Like, many others, I reacted more to my second dose. I had some fatigue and ran a fever several hours after I received the vaccine. Both were quickly remedied with a dose of Ibuprofen and sleep and didn’t last beyond the day I received the vaccine. I hope both my experience receiving the vaccine and my answers to your covid vaccine questions will help when you get the chance to receive your vaccine.

