by Christine Doronio Your blood is made up of four main components: red blood cells,…
Ask a Scientist: How do we know scientific studies are safe? Who approves them?
By Alexandra Wickson and Samantha Yeligar
Have you heard a lot about COVID-19 vaccine trials recently? Ever wonder who oversees and approves these trials to make sure they are safe and ethical?
Every single scientific study using people or animals must go through a review and approval process before the study can begin. This ensures the study will be ethical, safe, and that there are minimal risks or that the risks do not outweigh potential benefits. These review processes were established by law in 1974 in response to abusive and highly unethical experiments done in the 20th century (ex. Nazi physician experiments, the Tuskegee Syphilis Study).
What research requires review?
For studies involving humans, the review and approval process is done by an Institutional Review Board (IRB). For studies involving animals, this review and approval is done by the Institutional Animal Care and Use Committee (IACUC). Most human subjects research requires an IRB approval. There are a few types of research that don’t require an IRB due to minimal risk to the participant. These studies include:
- Instructional/curriculum related studies
- Analysis of existing data
- Studies involving possible changes to public service programs
- Food quality or taste studies
Everything else requires an IRB review.
Who is on an IRB?
IRBs can be established within government, universities, private research companies, or through an outside group that conducts the review on behalf of the institution doing the research.
An IRB board has specific requirements on who the members can be. Each IRB must have 5 members and must consider diversity in race, gender, and culture when choosing members. Additionally, the IRB must include at least:
- 1 member who works in a scientific area
- 1 who works in a nonscientific area
- 1 that is unaffiliated with the institution
What does the IRB Do?
The purpose of IRB review is to protect the rights and welfare of human subjects. The IRB reviews the research proposal and then approves, requests modifications, or rejects the proposal. IRBs do not evaluate or comment on the methods of the research unless it directly pertains to the human subjects’ welfare. The scientific merit of a study is judged via peer review and funding bodies.
What does the IRB review?
IRBs review all parts of the research that involve human interaction. This includes the entire study proposal, materials used for the study (surveys, questionnaires, trial medicines, etc.), information on how participants will be chosen, how participants will be told about the study and how they will consent to participate (called informed consent), and information pertaining to participant privacy and confidentiality. IRB requirements for approving research:
- Minimized risks to participants
- Risks are reasonable in relation to potential benefits
- Equitable selection of participants
- Informed consent will be given by each participant & documented
- Informed consent means that the participant not only agrees to participate in the research, but that they fully understand the risks, benefits, and requirements of the research.
- A plan for monitoring participant safety throughout the research, including safety metrics and thresholds that must be met in order for the research to continue
- There are adequate protections for participants related to privacy and confidentiality
How does this process apply to vaccines?
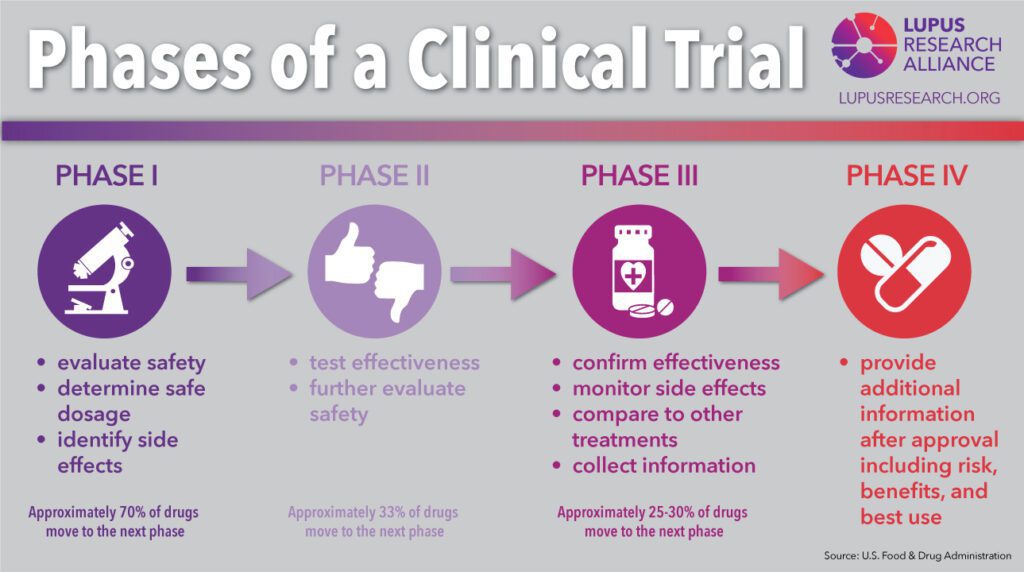
In addition to IRB approval, vaccines must also receive oversight from the FDA (Food & Drug Administration) throughout the development process. Vaccine development goes through 5 stages:
- The Preclinical Stage: Laboratory testing to show potential benefit and animal studies to ensure the vaccine is safe for human trials.
- Phase I Clinical Trials: Test the vaccine for safety by administering to a small number of human volunteers (~20-100 people).
- Phase II Clinical Trials: Test for short-term side effects and dosage needed for effectiveness by administering to a larger pool of volunteers (about a few hundred volunteers).
- Phase III Clinical Trials: Tests effectiveness of the vaccine and identify additional information related to safety and side effects by a blind trial. The volunteers (about a few hundred to a few thousand) are split into two group: one receives the vaccine the other receives a placebo. If there is no different between the groups, the vaccine is ineffective.
- Phase IV Clinical Trails: Tests effectiveness and side effects on a large scale. After FDA review, the vaccine is administered to thousands of volunteers.
During the trial, if the vaccine is found to harm participants or not work, the trial will be stopped immediately. The length of time each stage takes can depend on a number of factors, including funding, the number of people working on the research, and whether previous research on the drug or treatment already exists.
Take Action: Get your flu shot today! Be on the lookout for the Covid-19 vaccine.
More Information
For more on vaccine development, visit:
For more information on Institutional Review Boards, visit:
- FDA IRB FAQs
- Wikipedia IRB
- Wikipedia Belmont Report
- 45 CFR 46 (issued by the National Research Act of 1974, the way these regulations came to be law)


